Abstract
Background: Sickle cell disease (SCD) is an inherited chronic hematological disorder which causes significant morbidity and reduced life expectancy. For many patients with SCD, jaundice can be a significant clinical disease manifestation and may have an underappreciated impact on patient well-being and quality of life. We report a case of a patient with SCD and chronic jaundice treated with GBT440, a novel small molecule hemoglobin modifier and potential disease-modifying therapy for SCD. GBT440 is a potent anti-sickling agent, reduces hemolysis and has demonstrated good safety and tolerability.
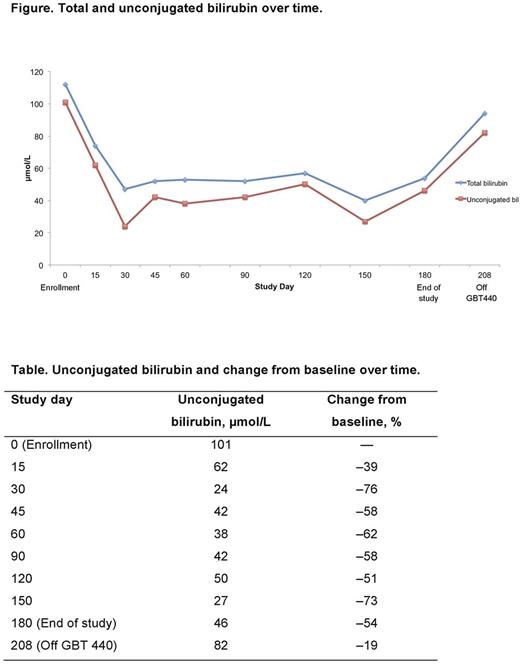
Case Presentation: A structured interview was conducted to provide the patient's description and experience with jaundice before, during, and after GBT440 treatment. The patient is a 27-year-old Black male diagnosed with SCD at birth who recalls learning about his SCD from the age of 7 years. In this patient, jaundice has manifested as scleral icterus. Relevant medical history in the previous 12 months as of March 2016 included 2 hospitalizations due to painful crises, no blood transfusions, and no treatment with hydroxyurea. SCD symptoms included pain, fatigue, yellow eyes, stress, and a feeling of being overwhelmed. Minor crises occurred 2-3 times per week. Total and unconjugated bilirubin at baseline were 112 and 101 µmol/L (6.5 and 5.9 mg/dL), respectively, and hemoglobin was 9.9 g/dL. The patient participated in two GBT440 trials; GBT440-001 in which he received placebo, and a follow-on extension, in which he received GBT440 900 mg orally once daily for 6 months.
Before GBT440 treatment, the patient reported that his eyes had been yellow since he was young. He reported no pain associated with jaundice and in general, the jaundice did not impact his daily life functioning and socializing; he stated he had learned to cope with it. However, he reported that his eyes did "go really, really yellow" when a crisis was coming. The patient wore tinted glasses, suggesting that he was bothered by his appearance and that others noticed it.
During GBT440 treatment, the patient's eyes cleared within 1 week of taking the study medication; his eyes were "back to normal with no sign of yellow" and stayed completely white throughout the trial. Friends and family noticed the change in his eyes and the patient stopped wearing tinted glasses. The patient reported that he had more energy and felt stronger, and the minor crises stopped. He also indicated "a sense of delight, much happier, great feeling". GBT440 treatment reduced bilirubin over time (Figure); unconjugated bilirubin was reduced by up to 76% (Table). The decline in unconjugated bilirubin in this patient is consistent with that previously reported (median decrease of 43% at the 900 mg/d dose; Howard et al. ASH 2016 meeting poster), and was below the bilirubin threshold typically associated with clinical jaundice (3 × upper limit of normal, 51 μmol/L) for most of the treatment period. In GBT440-001, for the patients dosed for ≥90 days, there were two additional patients with bilirubin elevated to the range of jaundice at baseline; they had a similar resolution of hyperbilirubinemia while receiving study drug. In this patient, hemoglobin improved from 9.9 g/dL at baseline to 11.1 g/dL at 90 days.
After stopping GBT440, the patient's eyes "returned to how they were before the trial, yellow", which started "as soon as I stopped the medicine". While receiving GBT440, the patient "felt so much better; the SCD pain was gone; felt happier, took away the stress and worrying about pain", and now feels worse while off the study medication. The patient rated the study medication as very beneficial. He indicated that "since birth, nothing has made my eyes go clear; I tried so many different things and they didn't help and within a short time on the medication, my eyes were clear".
Summary: The case patient had a long history of SCD with clinical jaundice and scleral icterus. He learned to cope with his jaundice so it did not significantly impact his activities of daily living. The patient reported that his jaundice cleared within 1 week of starting GBT440, that he felt much better with more energy, and that he was relieved/very happy after his eyes cleared. The jaundice returned after the patient stopped GBT440. Jaundice impacts many adults with SCD, significantly impacting self-image. GBT440 treatment was associated with reduced bilirubin levels and improved jaundice in our case patient.
Telfer: Kyora Kirin: Research Funding; Mundi Pharma: Research Funding; Bluebird Bio: Consultancy; Terumo: Honoraria; ApoPharma: Honoraria; Pfizer: Other: Participation in EDMC; Global Blood Therapeutics, Inc.: Consultancy; Novartis: Consultancy. Lehrer: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Agodoa: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Jurek: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Landsman: Global Blood Therapeutics, Inc.: Employment, Equity Ownership. Mant: Quintiles: Employment, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal